Breakthrough made in understanding drug resistance to Breast Cancer treatment
New protein discovery could change the way some cancers are treated
Breast cancer patients who have a hereditary form of the condition could have a protein that stops their bodies responding effectively to treatment, according to new research.
Up to 10 per cent of hereditary cases of breast cancer are due to the patient inheriting a faulty cancer-causing gene, such as mutated variations of the BRCA1 gene.
Breast cancer is the most common cancer in the UK and people with BRCA1 mutations are considered high risk, and often opt for prophylactic breast removal, like actor Angelina Jolie famously did in 2013.
New research outlined in a paper published in Nature Cell Biology, has now identified ‘Shieldin’ proteins which affect the body’s response to the treatment of breast cancers, specifically cancers caused by the BRCA1 gene, and could lead to a greater understanding of how to stop the body resisting these treatments.
The team of researchers working at the Wellcome Trust/Cancer Research UK Gurdon Institute recently developed a new, highly effective class of cancer therapy known as PARP inhibitors. Drug resistance is a common physical response, so establishing how resistance can develop became the new focus of the research. Dr Harveer Dev, Wellcome Clinical Fellow and PhD student at St John's College, was the lead author on the paper.
![]()
Growing cancer cells are surrounded by healthy cells, illustrating a primary tumor spreading to other parts of the body through the circulatory system.Credit: Darryl Leja, National Human Genome Research Institute, NIH
Using state-of-the-art CRISPR gene editing technology, they scanned the human genome for factors which, when mutated, could cause drug resistance in cells that lacked BRCA1. One of these factors was the previously uncharacterised ‘Shieldin’ complex, so-called because it shields the ends of broken DNA and regulates DNA repair. Testing the levels of Shieldin in tumours could help predict whether they will respond to anti-cancer drugs called PARP inhibitors.
The BRCA1 gene is critical for the body to be able to perform DNA repair known as homologous recombination (HR). This is counterbalanced by an opposing ‘error-prone’ repair pathway known as Non-homologous end-joining (NHEJ).
The researchers identified Shieldin as a new component of the NHEJ pathway. BRCA1-negative cells rely on this error-prone DNA repair pathway, which makes them susceptible to PARP inhibitors. If Shieldin is removed from these cells, the imbalance between the repair pathways is reversed, restoring the ability of the cell to perform DNA repair by HR, overcoming the toxicity of PARP inhibitors and therefore leading to drug resistance.
"As we improve our understanding, we should be able to better predict the responsiveness of an individual patient to treatment"
Professor Steve Jackson, whose led the research, said “there is a balance between repair factors like BRCA1 which mediate error-free DNA repair, and Shieldin which acts as a barrier and must be removed to allow accurate HR”.
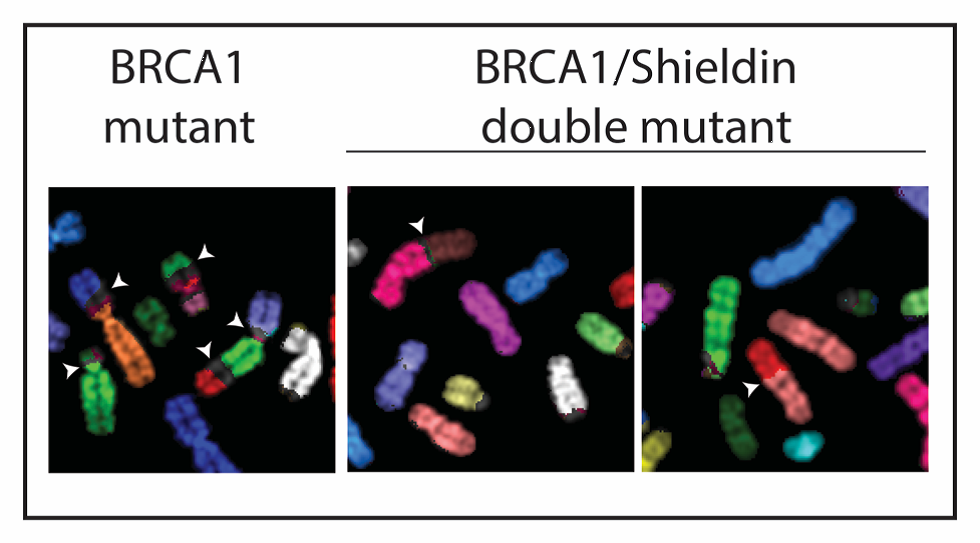
Loss of Shieldin restores genome stability in BRCA1 deficiency
Dr Dev explained: “In BRCA1 mutated cells, it appears as though the persistence of the Shieldin complex at DNA breaks renders these cells sensitive to PARP inhibitors. This explains why these drugs are effective in patients with BRCA1 mutations, but it also might help to explain how drug resistance can occur.”
The researchers went on to look at tumours from breast cancer patients carrying the BRCA1 mutation.
Dr Dev: "Those tumours with normal Shieldin levels initially respond to PARP inhibitors. However, if the Shieldin levels are low, or decrease following treatment, then resistance is observed. Hence testing the Shieldin status of BRCA1 mutated tumours might be useful in predicting responsiveness to this therapy.”
The study went on to show that resistance to PARP inhibitors can lead the same cancer cells to develop vulnerabilities to alternative cancer treatments, such as radiotherapy or platinum-based chemotherapy.
Professor Jackson concluded: “As we improve our understanding of these DNA repair networks and how they interact, we should be able to better predict the responsiveness of an individual patient’s tumour to specific therapies like PARP inhibitors, and ultimately personalise cancer therapy to achieve the maximal therapeutic response.”
Published: 23/07/18